|
|
|
|
|
|
| asdfadsfSulfonate
groupda |
Objective:
|
||||||
|
TRIBS
|
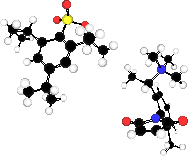 |
Trimethylammmonium |
|||||
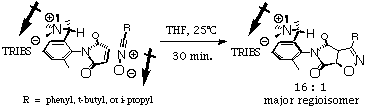 |
| Figure 2. 1,3-dipolar cycloaddition reaction. |
|
Progress: 3. Raposo, C.; C. S. Wilcox, C. S. "The Intramolecular Salt Effect in Chiral Auxiliaries. Enhanced diastereoselectivity in a nitrile oxide cycloaddition via rational transition state stabilization." Tetrahedron Lett. 1999, 1285-1288. |
[ Home | Related Sites | Members | Contact Us ]