Fractional distillation is a technique used to separate miscible liquids that have boiling point difference of less than 25 ºC.
SIMPLE vs. FRACTIONAL DISTILLATION
The only difference in apparatus between simple and fractional distillation methods is the use of a fractionating column used during fractional distillation. The fractionating column is placed directly above the boiling flask as shown below. The fractionating column may be packed with a variety of materials (a glass helices packed column is used in Chem0330).
COLUMN PACKING
Each type of packing has a known HETP (height equivalent to a theoretical plate) that is a measure of efficiency. The lower the HETP (measured in cm) the more 'theoretical plates' contained within a column. A theoretical plate is simply the distance it takes within the column for one complete vaporization-condensation cycle (or simple distillation) to occur. Because vaporization and condensation are equilibrium processes, the most important factor in carrying out a good fractional distillation experiment is heating slowly. This allows for equilibrium to be established throughout the column and the liquids contained in the mixture to separate. The best fractional distillation experiments have a high reflux ratio; liquid continually condenses back into the boiling flask
APPARATUS
Below is the setup for fractional distillation. It is similar to the apparatus for simple distillation (review that apparatus for more detail) except for the fractionating column.
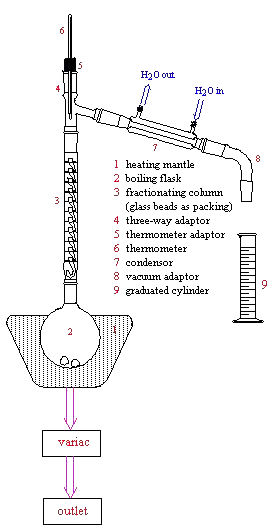
Below is a photograph of the actual fractional distillation apparatus used in Chem0330.

DATA
The same type of information can be collected for fractional distillation as was for simple distillation. However, fractional distillation is usually carried out to separate two miscible liquids. As the hot vapors pass through the column, they become successively more rich in the lower boiling component. This vapor is condensed and the 'fraction' collected. The temperature can be recorded to determine the compound's identity. This leaves the high boiling point component in the boiling flask. When enough energy is added to the boiling flask, the high boiling point component will boil, ascend the column as a gas, and condense as a second fraction. In this way, the two components can be isolated and their respective boiling points recorded for purposes of identification.
Below is a plot of the results from a fractional distillation. Each plateau indicates the boiling point of a component.
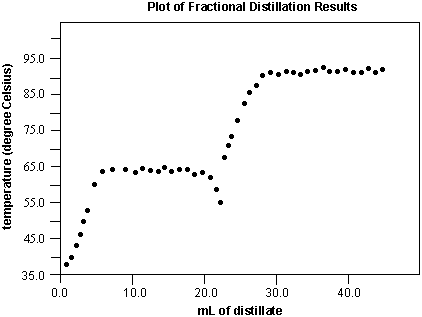
The identity of the each unknown can be determined. In this example, the low boiling point component is methanol (bp = 65.0 ºC) and the high boiling point component is isopropyl acetate (bp = 88.0 ºC). If 50 mL of the unknown mixture was distilled, then the mixture would have contained approximately 20 mL of methanol and 30 mL of isopropyl acetate. The decrease in temperature at 20 mL is a result of having completely distilled the methanol. The isopropyl acetate does not have enough energy to boil; its vapor has not reached the thermometer bulb and so the temperature the thermometer reads falls until this component reaches the bulb. If this occurs, increasing the setting on the variac will provide the necessary energy (in the form of heat) to distill the second component.