Column chromatography is one of the most important methods of separating (and purifying) solids and liquids. It is most often used on a small-scale (a few grams or mL of material), as the amount of chemical waste and time spent eluting the column increase as the amount of material increases. As with extraction, the fundamental concept utilized in column chromatography is polarity which determines the interactions of the sample molecules with the eluent and adsorbent.
ADSORBENT
The adsorbent is the stationary phase in column chromatography and fills the glass column. The common adsorbents used are alumina (Al2O3)and silica gel (SiO2). Both are polar. At Pitt, alumina is used to pack the column and provides the stationary phase upon which the sample adsorbs. Adsorption is the process of molecules 'adhering' to one another, without the making of chemical bonds.
ELUENT
The eluent is the mobile phase or the solvent that is passed through the column. Molecules in the sample will desorb off the adsorbent and dissolve in the eluent when the polarity of the eluent matches the polarity of the molecules. If a sample mixture contains just two compounds, one polar and the other nonpolar, then the elution process would begin by adding a nonpolar eluent (or solvent). As this solvent passes through the adsorbent, the nonpolar molecules in the sample dissolve in the nonpolar eluent and pass through the column. Once collected the eluent (now called 'eluant') contains the nonpolar sample molecules while the polar sample molecules remain adsorbed to the adsorbent. Passing a polar eluent through the column will dissolve the remaining polar molecules. By collecting the two resultant eluants in separate flasks, separation of the two compounds is achieved. Although column chromatography does not require colored compounds (it only requires that molecules in a mixture have differing polarity), the experiment performed at Pitt with plant pigments of spinach utilizes color to demonstrate the separation process.
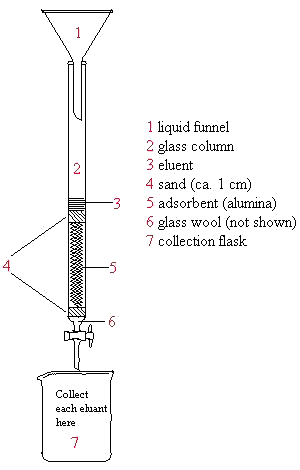
Below is an illustration of a packed column. Remember that the collection flask should be changed for each eluent used. Never let the column run dry and try not to disturb it after packing is complete. The lab manual (as your TA) provides step-by-step instruction for packing the column.