Research
 My long-term goal is to understand the relationship between behavior, cortical network activity and sensory perception. Although these investigations are often conducted in vivo, my approach is to use in vitro slice preparations of the olfactory system to directly measure the cellular and synaptic mechanisms that underlie cortical processing. A key aspect of my research is the use of electrophysiological, optogenetic and computational tools in the design of behaviorally relevant stimulus protocols. In addition, experimental results are incorporated in network models to connect cellular, synaptic and network properties to sensory processing at a systems level.
My long-term goal is to understand the relationship between behavior, cortical network activity and sensory perception. Although these investigations are often conducted in vivo, my approach is to use in vitro slice preparations of the olfactory system to directly measure the cellular and synaptic mechanisms that underlie cortical processing. A key aspect of my research is the use of electrophysiological, optogenetic and computational tools in the design of behaviorally relevant stimulus protocols. In addition, experimental results are incorporated in network models to connect cellular, synaptic and network properties to sensory processing at a systems level.
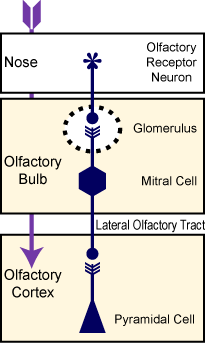
The organization of the olfactory system makes addressing the relationship between sensory input and cortical output highly tractable. The neural pathway between the nose and cortex is a short- consisting of just two synapses connecting the olfactory receptor neurons, mitral/tufted cells and cortical pyramidal cells. Both of these synapses can be maintained in a single brain slice consisting of the olfactory bulb and olfactory cortex. However, despite this seemingly simple architecture, the olfactory cortex is just now emerging as a model for sensory processing and essentially nothing is known about how respiratory behavior, such as sniffing, influences cortical activity.
Past work
Behaviorally relevant stimuli are differentially coded in pyramidal cell spike trains.During my doctoral studies with Dr. Leonard Maler at the University of Ottawa, I was introduced to a neuroethological approach to sensory processing. My research addressed how behaviorally relevant, prey and communication stimuli are represented in the electrosensory system of electric fish. These stimuli are directly relayed from the electroreceptors to intrinsically bursting pyramidal cells in the electrosensory lateral line lobe (ELL). In a number of sensory systems, bursts of spikes with short inter-spike intervals are thought to code specific stimulus features. To test this hypothesis, mimics of prey and communication stimuli were used to drive pyramidal neurons. By combining experimental and computational approaches, we found that intrinsic cellular properties allowed pyramidal neurons to encode prey stimuli in bursts of spikes and communication stimuli in isolated spikes (Oswald et al, 2004, 2007, Doiron et al.,2007). These studies brought two important perspectives to my current research: 1) the importance of using behaviorally relevant stimulus paradigms and 2) combining experimental and computational techniques is a powerful approach to studying sensory processing.
Cortical architecture, synaptic dynamics and gamma oscillations.
My doctoral work demonstrated that stimulus features could be encoded in patterns of spiking activity, leading to the question, " How do downstream neural networks decode these responses? " I joined the laboratory of Dr. Alex Reyes at New York University to answer this question by studying cortical processing in the auditory system. Cortical sensory processing has been classically divided into feed forward and recurrent network mechanisms. By recording large numbers of connected pairs, I analyzed the circuit architecture and the development of cellular and synaptic dynamics in the L2/3 pyramidal cell and fast spiking interneuron network (Oswald and Reyes, 2008, 2010). Incorporating these results in a cortical population model, we demonstrated that gamma oscillations are generated by recurrent interactions but the strength of the oscillation depends on spatial extent of feed forward network activation (Oswald et al., 2009). This suggests that stimulus characteristics that change the spatial profile of network activation can be represented by the degree of oscillatory population activity. This work has provided a strong foundation in cortical network analysis and processing that we use in current investigations in the olfactory cortex.
Active sniffing behavior gates intensity coding in the olfactory cortex.
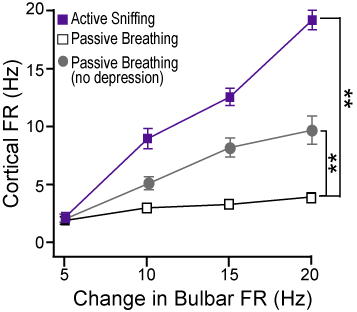 In the laboratory of Dr. Nathan Urban in the Department of Biological Sciences at Carnegie Mellon my projects addressed the mechanisms of information transfer between the olfactory bulb and the cortex. Analyses of neural coding generally focus on the relationship between stimulus and spike train statistics but make assumptions about how information is decoded between brain centers. Although information contained in spike trains must be transmitted through synapses between neurons, few studies have investigated a direct role for synapses in the coding of sensory information. It is commonly hypothesized that short-term synaptic plasticity filters information transfer between brain areas. I have tested this idea with respect to short-term plasticity at synapses between the olfactory bulb and cortex. Briefly, cortical pyramidal cells were driven by stimuli that incorporated the timescales of depression, the respiratory behavior of rodents and the changes in bulbar firing rates with odor intensity. The resulting cortical firing rates coded the change in stimulus intensity during active sniffing (right), but the recruitment of short-term depression produced invariant cortical responses during passive breathing (Oswald and Urban, 2012). This suggests animals may perceive changes in stimulus intensity during sniffing that are undetectable during passive breathing. This study shows how synaptic properties combined with a simple change in behavior can gate information transfer between olfactory brain centers.
In the laboratory of Dr. Nathan Urban in the Department of Biological Sciences at Carnegie Mellon my projects addressed the mechanisms of information transfer between the olfactory bulb and the cortex. Analyses of neural coding generally focus on the relationship between stimulus and spike train statistics but make assumptions about how information is decoded between brain centers. Although information contained in spike trains must be transmitted through synapses between neurons, few studies have investigated a direct role for synapses in the coding of sensory information. It is commonly hypothesized that short-term synaptic plasticity filters information transfer between brain areas. I have tested this idea with respect to short-term plasticity at synapses between the olfactory bulb and cortex. Briefly, cortical pyramidal cells were driven by stimuli that incorporated the timescales of depression, the respiratory behavior of rodents and the changes in bulbar firing rates with odor intensity. The resulting cortical firing rates coded the change in stimulus intensity during active sniffing (right), but the recruitment of short-term depression produced invariant cortical responses during passive breathing (Oswald and Urban, 2012). This suggests animals may perceive changes in stimulus intensity during sniffing that are undetectable during passive breathing. This study shows how synaptic properties combined with a simple change in behavior can gate information transfer between olfactory brain centers.
Current Research Projects
In the Oswald Laboratory we investigate how inputs from the olfactory bulb are represented and processed by the olfactory cortex. To smell an odor, one must inhale. Respiration is a periodic behavior that delivers odor information on different timescales during passive breathing versus sniffing. Thus, the activation of cortical circuits likely differs in these two states depending on the temporal dynamics of cellular and synaptic properties of the network. Although individual odorants evoke reproducible spatial and temporal patterns of cortical population activity, it is not known how cellular, synaptic and circuit properties shape these responses. There are at least two means by which odor specificity may be achieved, 1) the patterns of connectivity and dynamics of excitatory inputs arriving from the olfactory bulb and 2) the architecture and dynamics of feed forward and recurrent inhibitory networks. Brain slice preparations are ideal for these studies because they contain presynaptic olfactory bulb and postsynaptic cortical populations as well as the synaptic connections between these areas. We use multi-electrode whole-cell electrophysiology, calcium imaging, optogenetics and computational techniques to investigate how synaptic properties influence the spatiotemporal responses of cortical populations to bulbar inputs arriving at different respiration frequencies.Cortical population activity evoked by excitatory drive from the olfactory bulb.
Since the olfactory cortex lacks the functional columnar structure of other primary sensory cortices, the spatial profile of activation in the olfactory cortex may play a role in sensory processing that is akin to associational areas. One question we are addressing is whether or not cortical population activity differs when the olfactory bulb inputs are driven at frequencies consistent with active sniffing versus passive breathing. We are also investigating how behavioral state affects the representation of stimulus identity and/or quality.
Feed forward and recurrent inhibitory circuitry in the piriform cortex.
Excitatory inputs from the olfactory bulb make connections with interneurons and drive feed forward inhibition. In addition, excitatory and inhibitory neurons within the cortex are highly interconnected forming recurrent inhibitory networks. The recruitment of inhibitory subpopulations likely differs between early processing mediated by drive from the bulb (feed forward) and ongoing cortical activity (recurrent) such as oscillations and interactions with downstream brain centers. Ongoing studies in the lab are focused on attaining detailed descriptions of the architecture and synaptic properties of inhibitory circuits in the piriform cortex. Additional studies focus on the activation of interneuron classes during passive breathing or active sniffing.
