| The Michael Group University of Pittsburgh |
|---|
|
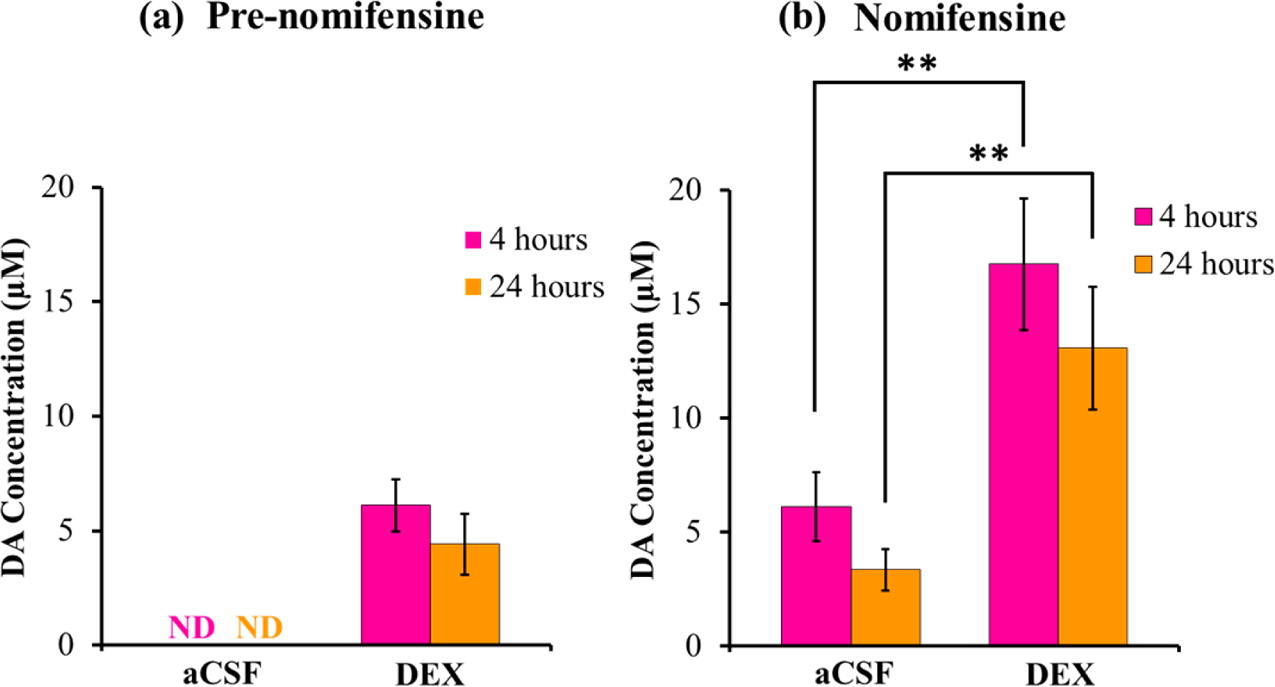
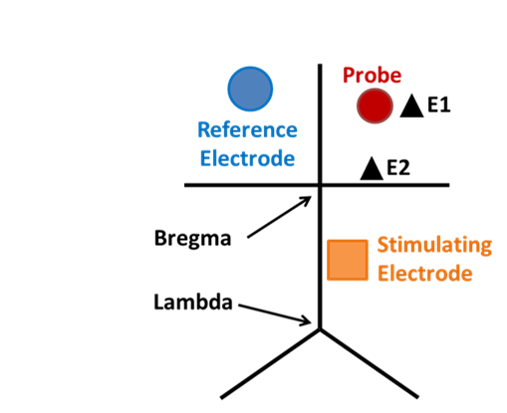
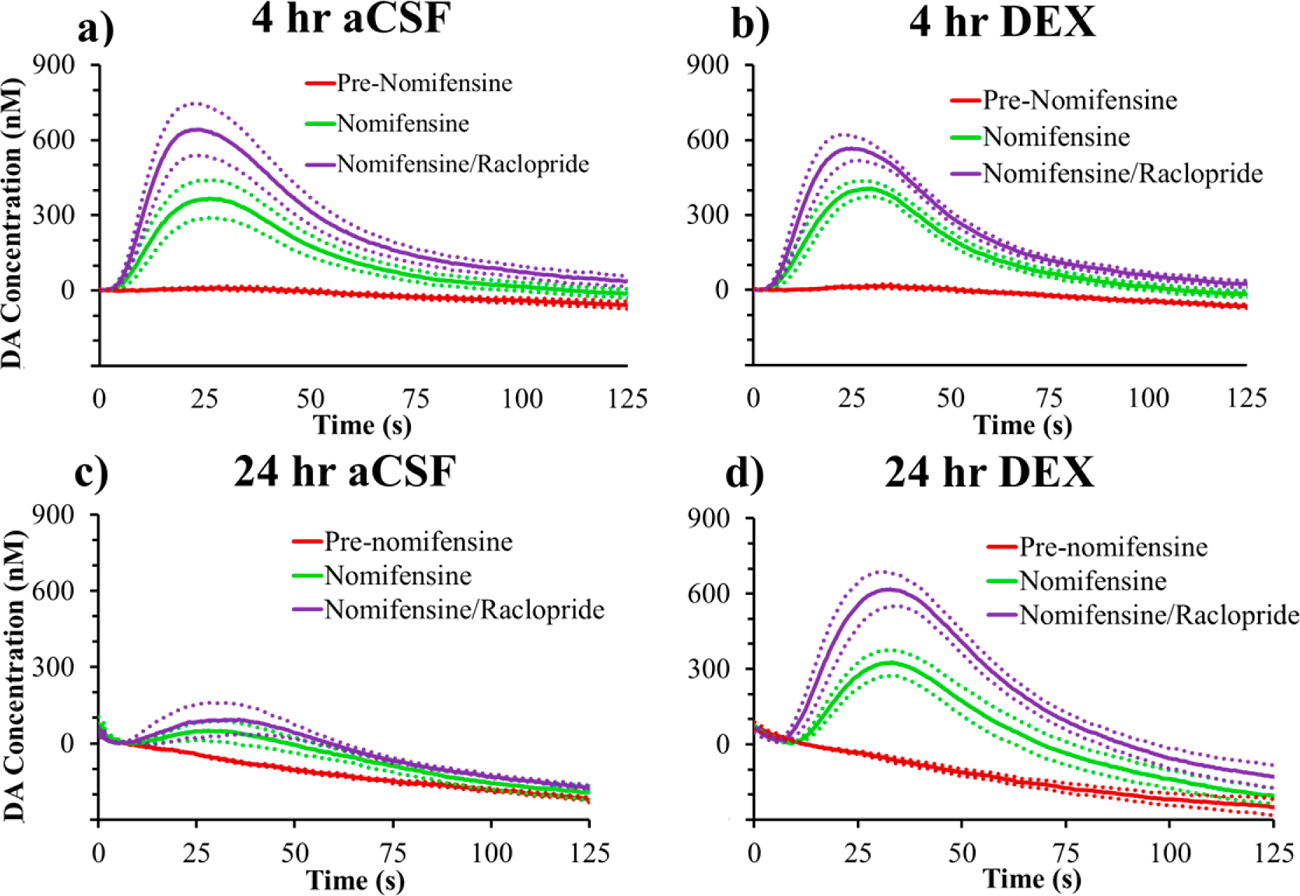
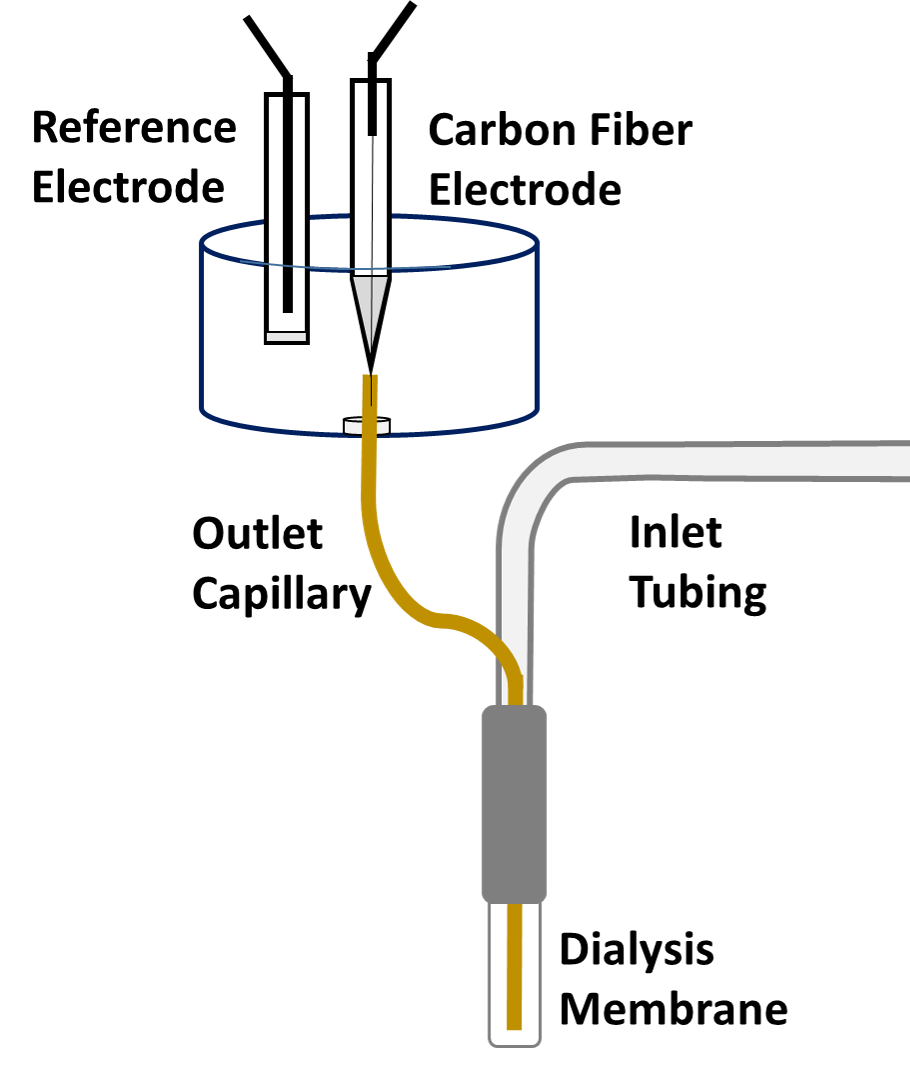
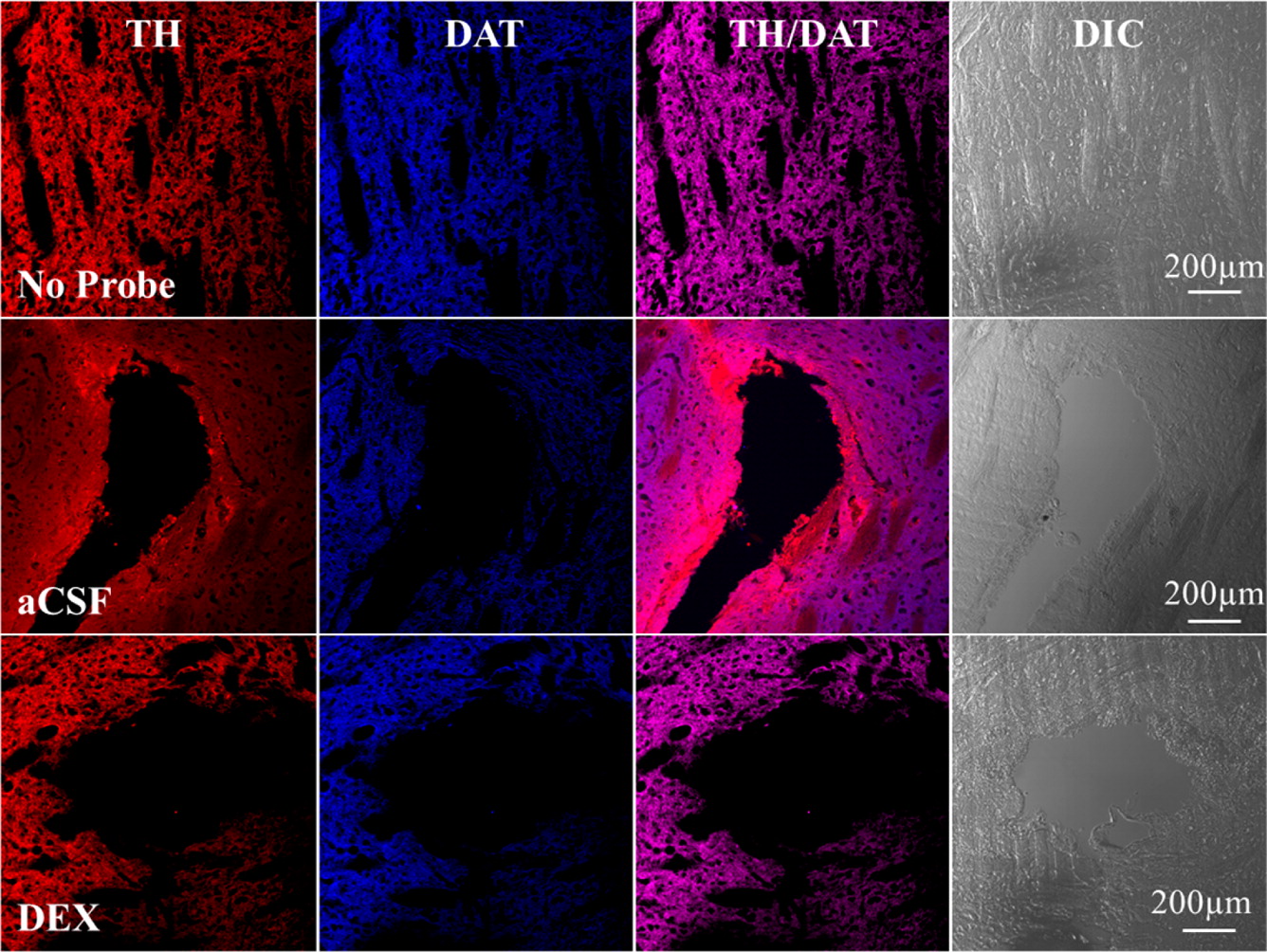
Microdialysis in the Rat Striatum: Effects of 24 h Dexamethasone Retrodialysis on Evoked Dopamine Release and Penetration Injury The power of microdialysis for in vivo neurochemical monitoring is a result of intense efforts to enhance microdialysis procedures, the probes themselves, and the analytical systems used for the analysis of dialysate samples. Our goal is to refine microdialysis further by focusing attention on what happens when the probes are implanted into brain tissue. It is broadly acknowledged that some tissue damage occurs, such that the tissue nearest the probes is disrupted from its normal state. We hypothesize that mitigating such disruption would refine microdialysis. Herein, we show that the addition of dexamethasone, an anti-inflammatory drug, to the perfusion fluid protects evoked dopamine responses as measured by fast-scan cyclic voltammetry next to the probes after 24 h. We also show that dexamethasone stabilizes evoked dopamine responses measured at the probe outlet over a 4–24 h postimplantation interval. The effects of dexamethasone are attributable to its anti-inflammatory actions, as dexamethasone had no significant effect on two histochemical markers for dopamine terminals, tyrosine hydroxylase and the dopamine transporter. Using histochemical assays, we confirmed that the actions of dexamethasone are tightly confined to the immediate, local vicinity of the probe.
|
|||||||